Stem cell transplant may be more effective than cyclophosphamide in treating scleroderma
- by Bianca Quijano
- Jan 15, 2018
- 2 min read
Myeloablative CD34+ autologous stem cell transplant is an effective treatment for severe scleroderma, otherwise known as diffuse cutaneous systemic sclerosis, according to a study published online ahead of print in The New England Journal of Medicine (Jan. 4, 2018).
The experimental treatment involves extracting hematopoietic stem cells from the patient’s blood which are stored in a freezer. Then, the patient’s malfunctioning immune system is destroyed through chemotherapy and radiation and the extracted stem cells transplanted back into the patient.
“This is a major advance in the treatment of severe scleroderma,” said Dr. Karen Ballen, co-author of the study and the director of Stem Cell Transplantation at the University of Virginia Cancer Center, in a press release.
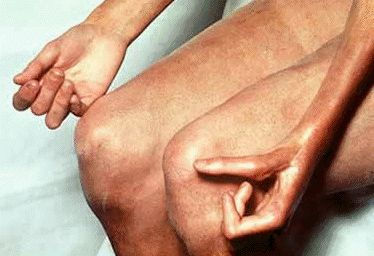
Thickened and tightened skin, primarily on the face, arms and hands resulting in loss of flexibility is a major symptom of scleroderma. Photo by the Mayo Foundation for Medical Education and Research.
Researchers tested the procedure in comparison to 12 monthly infusions of cyclophosphamide, the most effective existing treatment for the condition. Thirty-six adults with severe scleroderma underwent myeloablative autologous stem-cell transplantation while 39 other participants received cyclophosphamide.
After 72 months, 86% of those who received the stem-cell transplant remained alive, compared to 51% of those treated with cyclophosphamide.
Only 9% of the participants in the stem cell group had started using disease-modifying antirheumatic drugs at 54 months, in contrast with 44% of those in the cyclophosphamide group. It is important to note that treatment-related mortality in the stem cell transplantation group was 3% at 54 months and 6% at 72 months, compared to 0% at both time points in the cyclophosphamide group.

According to the researchers, risks of infections and low blood-cell counts were associated with both treatments. The overall infection rates were similar.
A global rank composite score among the participants was used to measure results on a basis of a hierarchy of disease features including: death, event-free survival (survival without respiratory, renal, or cardiac failure), forced vital capacity, score on the Disability Index of the Health Assessment Questionnaire, and modified Rodnan skin score.
The research was funded by the National Institutes of Health’s National Institute of Allergy and Infectious Diseases in the United States.
Dr. Karen Ballen, co-investigator, is also
director of the Cancer Centre at the
University of Virginia.
Photo by the University of Virginia
Health System.




Comments