Fibroblast variants identified—potential drug target for multiple diseases
- John Evans
- Sep 25, 2025
- 3 min read
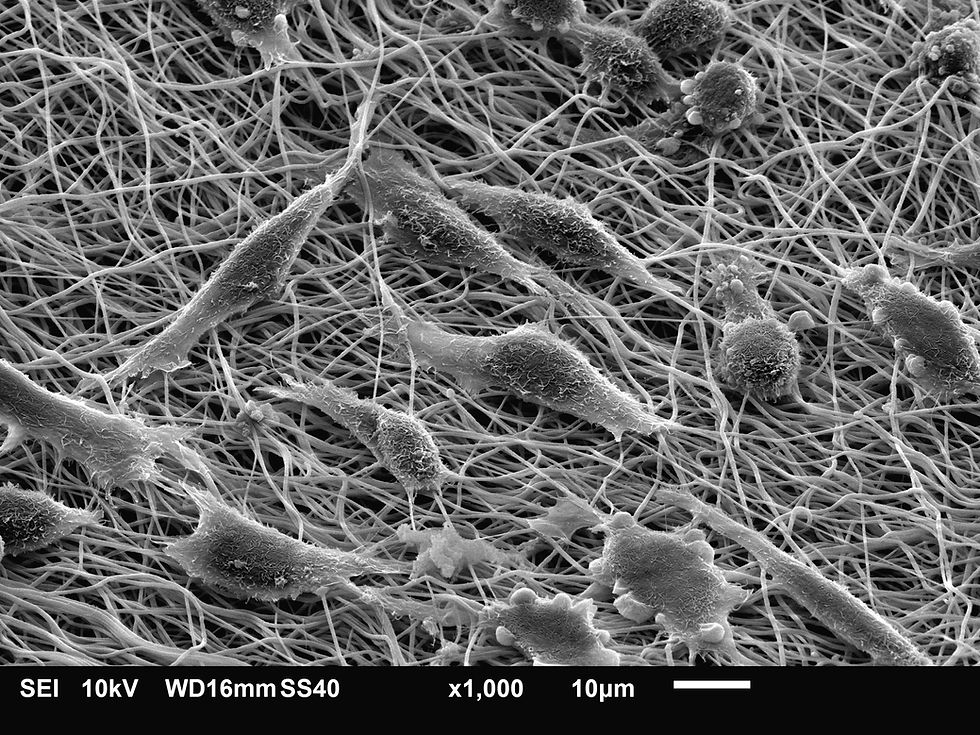
Scientists have identified eight different types of fibroblasts and how they form “tissue neighbourhoods” in the skin. The researchers also demonstrate how fibroblasts become “rogue” in various diseases affecting multiple organs, potentially representing a universal drug target.
Researchers at the Wellcome Sanger Institute, the University of Cambridge, and the University of Newcastle, along with their collaborators, published the findings in Nature Immunology.
This study is part of the international Human Cell Atlas (HCA) consortium, which is mapping all human cells to understand human health and aid in the diagnosis, monitoring, and treatment of diseases.
Fibroblasts, found in skin and every other organ in the body, are involved in wound healing, scarring, tissue repair, the development of connective tissues and the maintenance of skin.
In a press release, the authors note that skin fibroblasts have been somewhat overlooked, partly because their diversity has been challenging to study. While it is known that fibroblasts increase their collagen production and develop muscle-like fibres to contract after wounding, it has been unclear how fibroblast states change across the many diseases observed in skin, from cancers to acne.
In the new study, the investigators map fibroblasts in human skin, using healthy skin samples and those from 23 skin disorders, including psoriasis, lupus, skin cancers, and acne.
The team generated spatial transcriptomic data, measuring gene expression and its variation across different locations within a tissue, to spatially map the identified fibroblast populations in both normal and diseased human skin.
The team discovered five different types of fibroblasts in healthy skin, which are located in distinct “tissue neighbourhoods” associated with specific functions. Following this, the team examined fibroblasts and their tissue neighbourhoods in other organs, including the endometrium, gut, and lung in 14 diseases, such as inflammatory bowel disease and lung cancer. The team identified fibroblast populations shared across organs.
Using machine learning models, they identified three “rogue” subtypes of fibroblasts that are present in different organs across multiple diseases, including scarring diseases, lung cancer, rheumatoid arthritis in joints, and inflammatory bowel disease in the gut.
The same activated fibroblasts that recruit immune cells to early skin wounds were observed in inflammatory diseases, such as acne and inflammatory bowel disease. This observation suggests that a wound-like fibroblast state is used to attract immune cells to tissues in these conditions.
By identifying shared disease-related and disease-specific fibroblasts within these tissue neighbourhoods across multiple diseases and organs, the researchers say they have identified potential universal drug targets, allowing for the development of medications that work for several diseases across the body.
“Fibroblasts have critical roles in recruiting immune cells to skin tissue and causing scarring, which can result in a range of skin diseases. We lack highly effective treatments to treat scarring in clinical practice, partly because these cells have been poorly understood,” said first author Dr. Lloyd Steele, in the release. “Modern technologies now allow us to begin to understand these critical cells in unprecedented detail. We’ve shown for the first time that fibroblasts occupy and maintain distinct anatomical microenvironments in skin tissue in health and disease. Our data are freely available, and we provide an online tool that can be used by researchers to map fibroblasts from their own studies, to expand our knowledge on fibroblasts in disease.”
Dr. Steele is a researcher at the Wellcome Sanger Institute.
The team aims to extend this research to many other cell types across all tissues in the human body and utilize machine learning and artificial intelligence to identify disease-specific tissue neighbourhoods that can be modulated for therapeutic purposes.




Comments